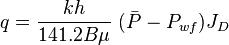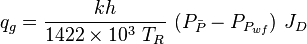Difference between revisions of "Vogel's IPR"
From wiki.pengtools.com
(→Vogel's Inflow Performance Relationship) |
(→Vogel's Inflow Performance Relationship) |
||
| Line 6: | Line 6: | ||
[[Vogel's IPR]] is based on computer simulations to several solution gas drive reservoirs for different fluid and reservoir relative permeability properties. | [[Vogel's IPR]] is based on computer simulations to several solution gas drive reservoirs for different fluid and reservoir relative permeability properties. | ||
| + | |||
| + | [[Vogel's IPR]] solution has been found to be very good and is widely used in prediction of IPR curves <ref name=KB1984 />. | ||
==Math and Physics== | ==Math and Physics== | ||
Revision as of 08:13, 5 April 2019
Contents
Vogel's Inflow Performance Relationship
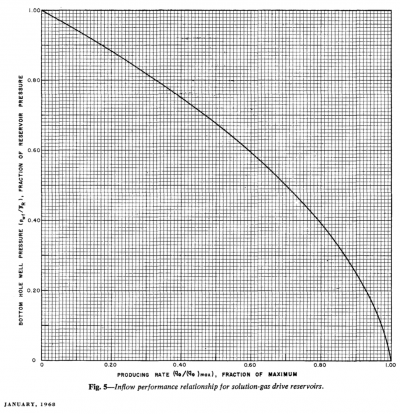
Vogel's IPR[1]
Vogel's IPR is an empirical two-phase (oil + gas) inflow performance relationship correlation published in 1968 [1].
Vogel's IPR is based on computer simulations to several solution gas drive reservoirs for different fluid and reservoir relative permeability properties.
Vogel's IPR solution has been found to be very good and is widely used in prediction of IPR curves [2].
Math and Physics
Oil well IPR equation
- Darcy's law inflow equation for the single phase incompressible liquid:
- Vogel's IPR two phase equation (oil + gas) and it's combination with single phase liquid
- Composite IPR three phase equation (oil + gas + water)
Gas well IPR equation
- Darcy's law gas inflow equation:
- C and n equation
IPR calculator software
- PQplot nodal analysis software is used to calculate the IPR curves. PQplot is available online at www.pengtools.com.
- Excel
- other
Nomenclature
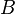 = formation volume factor, bbl/stb
= formation volume factor, bbl/stb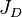 = dimensionless productivity index, dimensionless
= dimensionless productivity index, dimensionless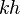 = permeability times thickness, md*ft
= permeability times thickness, md*ft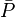 = average reservoir pressure, psia
= average reservoir pressure, psia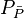 = average reservoir pseudopressure, psia2/cP
= average reservoir pseudopressure, psia2/cP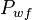 = well flowing pressure, psia
= well flowing pressure, psia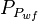 = average well flowing pseudopressure, psia2/cP
= average well flowing pseudopressure, psia2/cP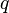 = flowing rate, stb/d
= flowing rate, stb/d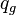 = gas rate, MMscfd
= gas rate, MMscfd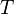 = temperature, °R
= temperature, °R
Greek symbols
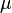 = viscosity, cp
= viscosity, cp