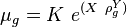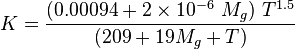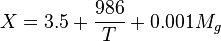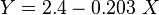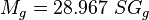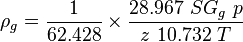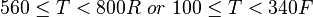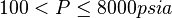Difference between revisions of "Lee correlation"
From wiki.pengtools.com
(→Math & Physics) |
(→Nomenclature) |
||
| Line 32: | Line 32: | ||
:<math> \rho_g </math> = gas density, g/cm3 | :<math> \rho_g </math> = gas density, g/cm3 | ||
:<math> \mu_g </math> = gas viscosity, cp | :<math> \mu_g </math> = gas viscosity, cp | ||
| − | :<math> M_g </math> = gas molecular weight | + | :<math> M_g </math> = gas molecular weight, dimensionless |
:<math> p </math> = pressure, psia | :<math> p </math> = pressure, psia | ||
:<math> SG_g </math> = gas specific gravity, dimensionless | :<math> SG_g </math> = gas specific gravity, dimensionless | ||
Revision as of 14:26, 2 May 2017
Brief
Lee correlation for viscosity of natural gases.
Math & Physics
where
Discussion
Why the Lee correlation?
Application range
Nomenclature
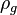 = gas density, g/cm3
= gas density, g/cm3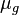 = gas viscosity, cp
= gas viscosity, cp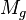 = gas molecular weight, dimensionless
= gas molecular weight, dimensionless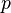 = pressure, psia
= pressure, psia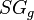 = gas specific gravity, dimensionless
= gas specific gravity, dimensionless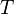 = temperature, °R
= temperature, °R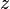 = gas compressibility factor, dimensionless
= gas compressibility factor, dimensionless
References
- ↑ Lee, A. B.; Gonzalez, M. H.; Eakin, B. E. (1966). "The Viscosity of Natural Gases". J Pet Technol (SPE-1340-PA).