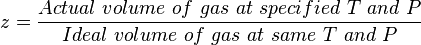Difference between revisions of "Gas compressibility factor"
From wiki.pengtools.com
| Line 3: | Line 3: | ||
[[gas compressibility factor]] is the ratio of the [[Real Gas]] volume to the [[Ideal Gas]] volume, which is a measure of the amount that the gas deviates from the perfect behavior. | [[gas compressibility factor]] is the ratio of the [[Real Gas]] volume to the [[Ideal Gas]] volume, which is a measure of the amount that the gas deviates from the perfect behavior. | ||
| − | :<math> z = \frac{Actual\ volume\ of\ gas\ at\ specified\ T\ and\ P}{Ideal\ volume\ of\ gas\ at\ same\ T\ and\ P} </math> | + | :<math> z = \frac{Actual\ volume\ of\ gas\ at\ specified\ T\ and\ P}{Ideal\ volume\ of\ gas\ at\ same\ T\ and\ P} </math> <ref name=PEHvol1 /> |
== Nomenclature == | == Nomenclature == | ||
| Line 10: | Line 10: | ||
:<math> T </math> = temperature, °R | :<math> T </math> = temperature, °R | ||
:<math> z </math> = gas compressibility factor, dimensionless | :<math> z </math> = gas compressibility factor, dimensionless | ||
| + | |||
| + | == References == | ||
| + | <references> | ||
| + | |||
| + | <ref name=PEHvol1>{{cite book | ||
| + | |last1= Fanchi |first1=John R. | ||
| + | |title=Petroleum Engineering Handbook, Volume I: General Engineering | ||
| + | |date=2006 | ||
| + | |publisher=SPE | ||
| + | |volume=1 | ||
| + | |ISBN=978-1-55563-108-6 | ||
| + | |||
| + | }}</ref> | ||
| + | |||
| + | </references> | ||
[[Category:pengtools]] | [[Category:pengtools]] | ||
[[Category:E&P Portal]] | [[Category:E&P Portal]] | ||
Revision as of 11:53, 20 November 2017
Brief
gas compressibility factor is the ratio of the Real Gas volume to the Ideal Gas volume, which is a measure of the amount that the gas deviates from the perfect behavior.
Nomenclature
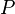 = Pressure, psia
= Pressure, psia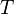 = temperature, °R
= temperature, °R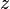 = gas compressibility factor, dimensionless
= gas compressibility factor, dimensionless
References
- ↑ Fanchi, John R. (2006). Petroleum Engineering Handbook, Volume I: General Engineering. 1. SPE. ISBN 978-1-55563-108-6.