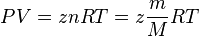Difference between revisions of "Real Gas"
From wiki.pengtools.com
(→Brief) |
(→Equation Of State) |
||
| Line 7: | Line 7: | ||
== Equation Of State == | == Equation Of State == | ||
| − | + | The [[Real Gas]] [[EOS]]: | |
| − | + | :<math> PV = zn RT = z\frac{m}{M} RT</math> | |
| − | |||
| − | :<math> PV = | ||
== Nomenclature == | == Nomenclature == | ||
Revision as of 11:39, 20 November 2017
Brief
Real Gas is a gas whose molecules occupy space and have interactions [1].
Real Gas deviates from Ideal Gas by proportionality term z, which is gas compressibility factor.
Equation Of State
The Real Gas EOS:
Nomenclature
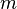 = mass, lbm
= mass, lbm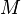 = molecular weight, lbm/lbmol
= molecular weight, lbm/lbmol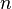 = number of moles, lbmol
= number of moles, lbmol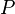 = Pressure, psia
= Pressure, psia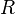 = universal gas constant, 10.7316 psia ft3/ lbmol/ °R
= universal gas constant, 10.7316 psia ft3/ lbmol/ °R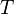 = temperature, °R
= temperature, °R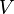 = volume, ft3
= volume, ft3