Difference between revisions of "Yield"
From wiki.pengtools.com
(→Brief) |
(→Nomenclature) |
||
| Line 13: | Line 13: | ||
== Nomenclature == | == Nomenclature == | ||
| − | :<math> | + | :<math> M_L </math> = liquid molecular weight, lb<sub>m</sub>/lb<sub>mol</sub> |
| + | :<math> \rho_L </math> = liquid density, lb<sub>m</sub>/ft<sup>3</sup> | ||
:<math> x </math> = molar fraction of the liquid phase, lb<sub>mol</sub> | :<math> x </math> = molar fraction of the liquid phase, lb<sub>mol</sub> | ||
:<math> y </math> = molar fraction of the vapor phase, lb<sub>mol</sub> | :<math> y </math> = molar fraction of the vapor phase, lb<sub>mol</sub> | ||
:<math> Yield </math> = yield, bbl/MMscf | :<math> Yield </math> = yield, bbl/MMscf | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
===Conversions=== | ===Conversions=== | ||
Revision as of 13:58, 20 April 2017
Brief
Yield is how much liquid hydrocarbons you get per volume of the gas produced.
Measured at the surface.
Calculated from the Flash.
Math & Physics
Nomenclature
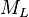 = liquid molecular weight, lbm/lbmol
= liquid molecular weight, lbm/lbmol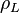 = liquid density, lbm/ft3
= liquid density, lbm/ft3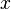 = molar fraction of the liquid phase, lbmol
= molar fraction of the liquid phase, lbmol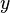 = molar fraction of the vapor phase, lbmol
= molar fraction of the vapor phase, lbmol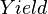 = yield, bbl/MMscf
= yield, bbl/MMscf
Conversions
379.5 scf/lbmol at Psc=14.7 psia and Tsc 60°F [1]
References
- ↑ John M. Campbell and Company (2004). Gas Conditioning and Processing. 1 The Basic Principles (8 ed.). Norman, Oklahoma, USA: John M. Campbell and Company. ISBN 0-9703449-0-2.
![Yield = 10^6 \times \frac{x \times M_L \times \frac{1}{\rho_L} \times \frac{1}{5.615}}{y \times 379.5}\ \left [ \frac{bbl}{MMscf} \right ]](/images/math/a/4/c/a4c06b66af9cf7b2766c531277bef949.png)
