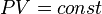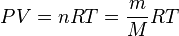Difference between revisions of "Ideal Gas"
From wiki.pengtools.com
(→Nomenclature) |
(→Equation Of State) |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Brief == | == Brief == | ||
| − | [[Ideal Gas]] is a concept where: | + | [[Ideal Gas]] is a [https://en.wikipedia.org/wiki/Ideal_gas concept] where: |
* gas is composed with molecules of negligible volume; | * gas is composed with molecules of negligible volume; | ||
* interaction between the molecules is negligible. | * interaction between the molecules is negligible. | ||
| Line 8: | Line 8: | ||
It all started with [https://en.wikipedia.org/wiki/Boyle%27s_law Boyle-Charles Law] published in '''1662''' . | It all started with [https://en.wikipedia.org/wiki/Boyle%27s_law Boyle-Charles Law] published in '''1662''' . | ||
| + | |||
| + | :<math> PV = const</math> | ||
The [[Ideal Gas]] [[EOS]]: | The [[Ideal Gas]] [[EOS]]: | ||
| − | :<math> PV = \frac{m}{M} RT</math> | + | :<math> PV = n RT = \frac{m}{M} RT</math> |
== Nomenclature == | == Nomenclature == | ||
| Line 17: | Line 19: | ||
:<math> m </math> = mass, lb<sub>m</sub> | :<math> m </math> = mass, lb<sub>m</sub> | ||
:<math> M </math> = molecular weight, lb<sub>m</sub>/lb<sub>mol</sub> | :<math> M </math> = molecular weight, lb<sub>m</sub>/lb<sub>mol</sub> | ||
| + | :<math> n </math> = number of moles, lb<sub>mol</sub> | ||
:<math> P </math> = Pressure, psia | :<math> P </math> = Pressure, psia | ||
| − | :<math> R </math> = universal gas constant, 10. | + | :<math> R </math> = universal gas constant, 10.7316 psia ft<sup>3</sup>/ lb<sub>mol</sub>/ °R |
:<math> T </math> = temperature, °R | :<math> T </math> = temperature, °R | ||
:<math> V </math> = volume, ft<sup>3</sup> | :<math> V </math> = volume, ft<sup>3</sup> | ||
| + | |||
| + | === See also === | ||
| + | [[Ideal Gas]]<BR/> | ||
| + | [[Real Gas]]<BR/> | ||
| + | [[gas compressibility factor]]<BR/> | ||
| + | [[EOS]]<BR/> | ||
| + | |||
| + | [[Category:E&P Portal]] | ||
Latest revision as of 07:52, 21 November 2017
Brief
Ideal Gas is a concept where:
- gas is composed with molecules of negligible volume;
- interaction between the molecules is negligible.
Equation Of State
It all started with Boyle-Charles Law published in 1662 .
The Ideal Gas EOS:
Nomenclature
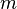 = mass, lbm
= mass, lbm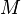 = molecular weight, lbm/lbmol
= molecular weight, lbm/lbmol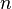 = number of moles, lbmol
= number of moles, lbmol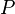 = Pressure, psia
= Pressure, psia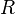 = universal gas constant, 10.7316 psia ft3/ lbmol/ °R
= universal gas constant, 10.7316 psia ft3/ lbmol/ °R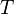 = temperature, °R
= temperature, °R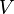 = volume, ft3
= volume, ft3
See also
Ideal Gas
Real Gas
gas compressibility factor
EOS